The main focus of the lab is to try and understand the neural mechanisms that support our ability to remember the things that have happened to us – episodic memory. Most of our work takes a systems neuroscience approach to examine how networks within the hippocampus and entorhinal cortex process memory information. We use in vivo electrophysiology to examine firing patterns of individual neurons as lab rats carry out memory tasks. We also use molecular and genetic tools to manipulate the network and understand the cellular mechanisms underlying episodic memory.
Complimenting the neuroscientific approach, other lines of research in the lab examine the cognitive mechanisms underlying episodic memory in both human adults and children. Ultimately we aim to apply this work by using our knowledge of mammalian memory networks to help test therapeutic strategies for disorders of memory such as Alzheimer’s disease. Current on-going research projects in the lab include:

Lateral Entrohinal Cortex and Associative Recognition Memory
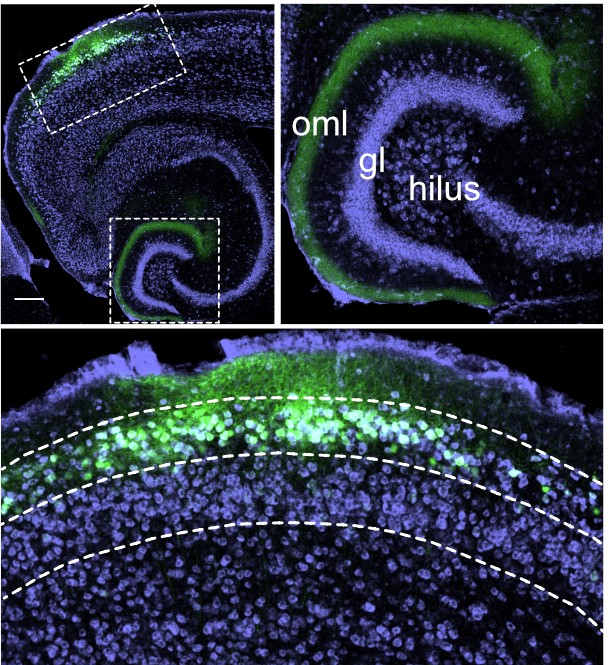
While it is well known that the hippocampus supports episodic memory in humans and animals our understanding of how the network of structures surrounding the hippocampus contributes to this cognitive function is less well understood. In recent years we have been using in vivo single unit recording and immediate early gene imaging studies combined with molecular and genetic tools to examine the role of one of the main interfaces between the hippocampus and neocortex, the lateral entorhinal cortex (LEC).
Our studies suggest a new role for LEC in associative memory for combinations of features of an event. Trying to remember where you left your keys or where you parked your car? Your LEC will be helping you remember the combination of object and the context in which it was experienced.
LEC and Spatial Cognition
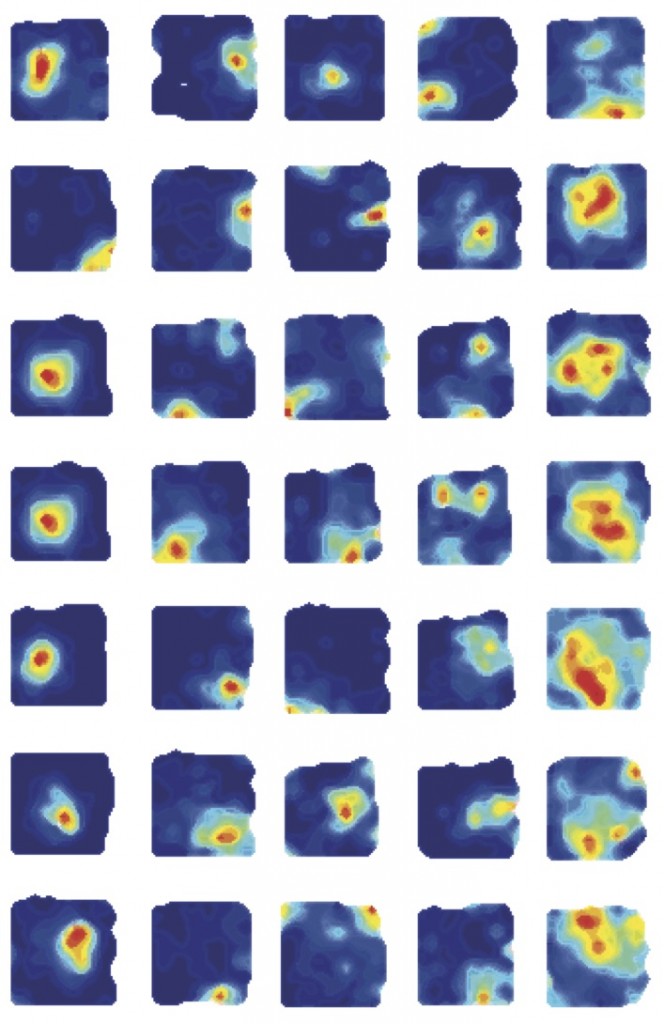
How do we know where we are? How do find our way around in familiar places? The answer to these questions is that we use cognitive maps which give us mental representations of places we have visited. The hippocampus is critically involved in cognitive mapping and place cells (see examples at the top of the page) are the basic building block of cognitive maps.
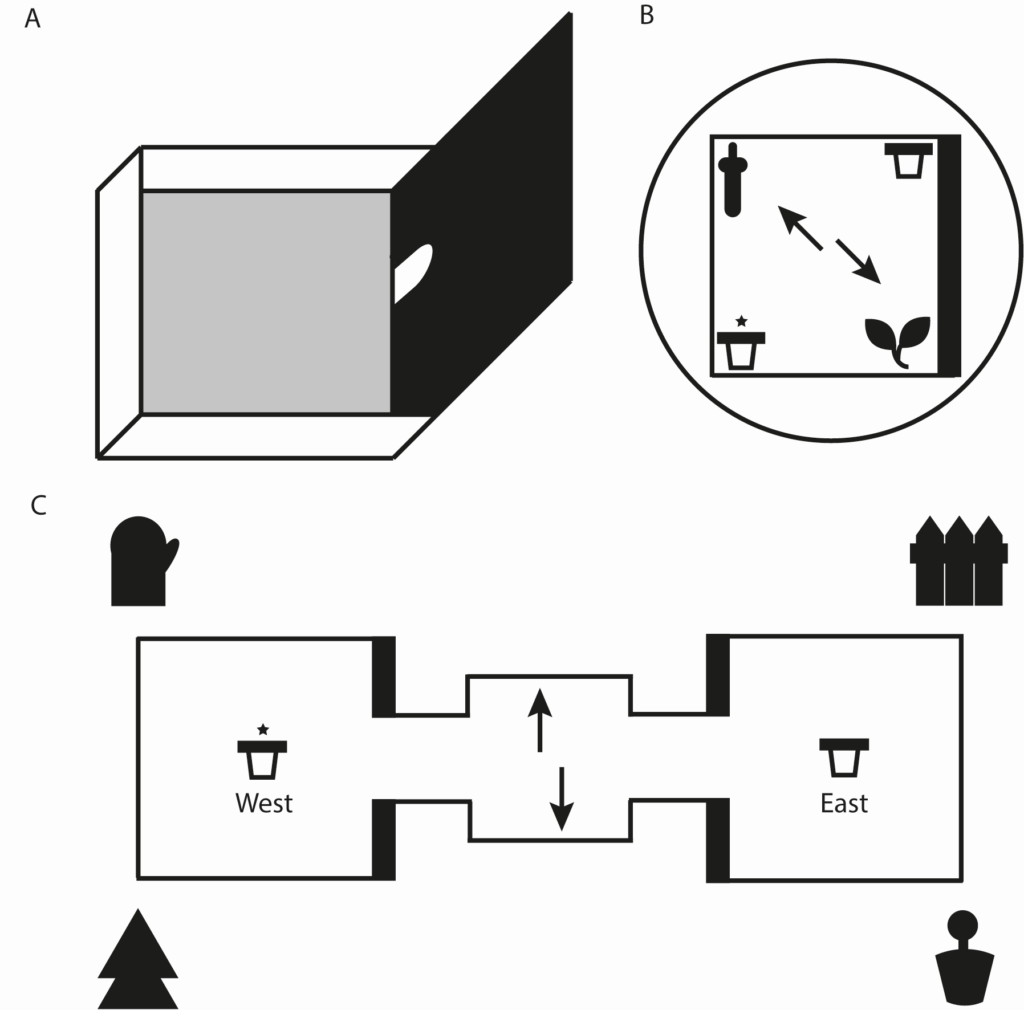
In recent years we have explored how inputs from LEC to the hippocampus contribute to our ability to know where we are. We have designed a number of behavioural tasks that examine the ability of rats and mice to navigate using different types of spatial cues. We have shown that LEC is important for understanding local features/cues that we can interact with but less so for large scale global features/cues. LEC is also important for first person (egocentric) spatial frameworks as well as world centred (allocentric) frameworks.
Consistent with findings covered above LEC is critical for allowing us to associate multiple features of environment together.
LEC input creates more precise memory for object locations
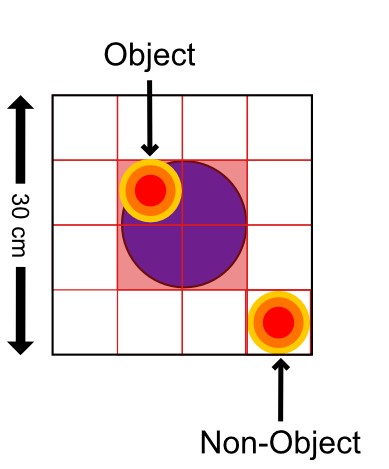
Place cells receive input from both lateral (LEC) and medial entorhinal cortex (MEC). However, place cells in some parts of the hippocampus receive much more input from LEC and other receive more input from MEC. Given our work showing that LEC is critical for integrating information about objects and the locations in which they are experienced, Vandrey et al. 2021 examined whether place cells getting LEC input are especially sensitive to object locations.
We found that place cells receiving LEC inputs have more precise spatial firing and also fire closer to objects. We also report object memory cells that encode where objects have been previously experienced. Object memory cells that receive LEC input are also more precise than those receiving MEC input.
Time vs. Context
How do we remember specific events? How is it possible that we can remember hundreds of very similar events that occur in the same location (how many meetings have I had in my office?!)? One way of disambiguating events is to encode the time at which they happened as this is a unique piece of information that can separate each memory from other memories. However, humans are not very good at remembering specific times and dates and as such we rely on other contextual cues that were present during the event (who was there, what the weather was like). Current experiments are asking which neural mechanisms allow is to remember time and contextual information from specific experiences.
Our recent findings call into question the suggestion that context and time are used interchangbly to remember episodes by showing that they can be supported by different retrieval mechanisms.
Translating rodent and human memory research
One very commonly used test of memory in lab rats is the novel object recognition paradigm. This works by making use of rodents’ natural propensity to explore novel objects and assumes that memory for familiar objects supports this novelty seeking behaviour. However, the translation of findings from these rodent studies relies on the assumption that humans and rats use the same memory mechanisms when carrying out this type of task. We are adapting rodent tasks for humans and vice versa to ask whether we are testing the same cognitive processes in both species.
Some of our recent work in humans using eye tracking and standard recognition memory protocols has validated the novel object recognition paradigm by demonstrating it is well correlated with memory sensitivity and not bias.
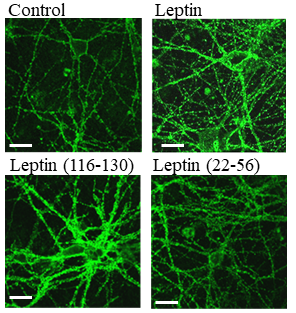
Leptin and Episodic Memory
Recent studies have suggested that irregularities in the system controlling the hormone leptin are linked with Alzheimer’s disease (AD). Leptin levels are reduced in AD patients and leptin has been shown to reduce b-amyloid (Ab) and tau phosphorylation, 2 hallmarks of AD pathology, in animal models of AD. However, we do not know how leptin affects the debilitating cognitive decline in AD which is characterised by deficits in memory, attention, language and spatial orientation in its final stages.
Our recent experiments have shown that leptin upregulates memory mechanisms within the hippocampus and improves episodic memory in mice. On-going studies are examining whether small fragments of the leptin molecule could be a useful therapeutic target for AD.